This is a work in progress. Contributions are welcome! Just click the edit button in the lower right corner. It's our goal to avoid any duplication of effort. So please include existing projects that would be interested in fulfilling any part of this global framework.
¶ Mandate
Maximize average healthy human lifespan and minizmize net suffering by identifying discovering and disseminating the effects of every food, additive, supplement, and medical intervention.
¶ Overview
The Wikipedia model demonstrates the tremendous power of crowdsourcing and open collaboration. Despite Microsoft spending billions of dollars hiring thousands of expert PhDs to create Encarta, Wikipedia garnered over 50 times more content in just a few years - all from volunteers. Moreover, studies have shown Wikipedia's accuracy on scientific topics rivals leading encyclopedias.
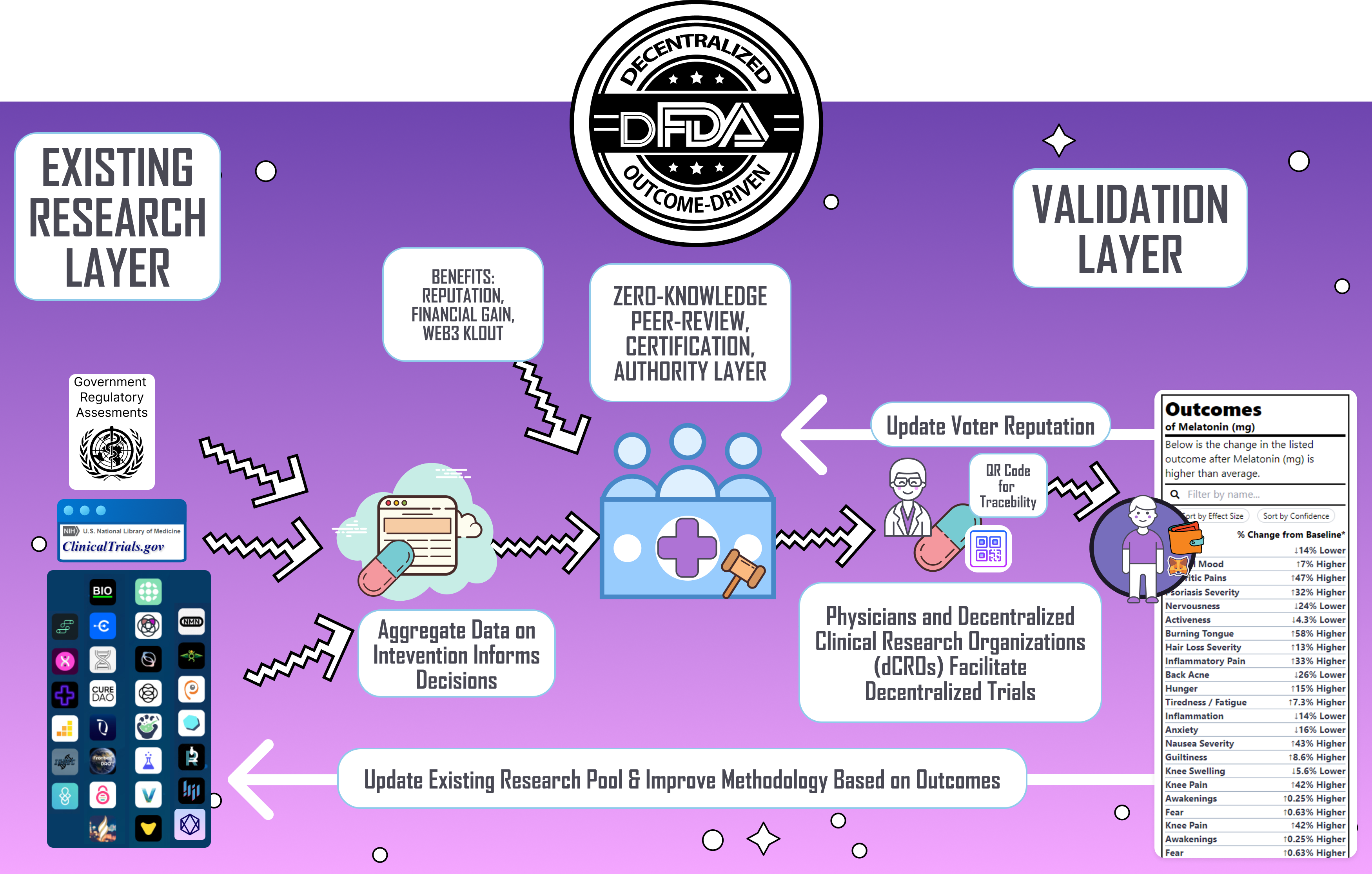
The Decentralized FDA aims to produce a 50X accerleration in clinical discovery by replicating this model for clinical research. By crowdsourcing real-world data and observations from patients, clinicians, and researchers, the Decentralized FDA could enable orders of magnitude more insights and discovery than the current closed research system.
Rather than a few expensive clinical trials conducted by pharmaceutical companies, the Decentralized FDA would facilitate a massive decentralized clinical trial encompassing millions of patients. This "Wikipedization" of evidence-based research can unlock lifesaving knowledge at a fraction of the cost and time of traditional methods. Just as Wikipedia democratized access to knowledge, the Decentralized FDA can democratize health research and empower people with actionable data to improve lives.
¶ Problems We're Trying To Solve Here
The existing regulatory framework for drug approval presents several challenges, hindering the efficiency and effectiveness of pharmaceutical research and public health advancements:
- Prolonged Time and High Costs: It takes over a decade and $2.6 billion on average to bring a drug to market, with Phase III clinical trials costing around $41k per subject.
- Limited Knowledge on Unpatentable Molecules: There is a significant lack of data on the long-term effects of the majority of synthetic or natural compounds, as there is insufficient incentive to research non-patentable molecules.
- Inadequate Exploration of Off-Patent Treatments: There is a lack of financial incentive to approve drugs for additional conditions after their patent expires, leaving potential treatments for rare diseases unexplored.
- Absence of Long-Term Outcome Data: Due to financial constraints, long-term effects of drugs are often unknown.
- Publication Bias: Negative results are frequently unreported, leading to redundant research efforts and wasted resources.
- Exclusion of Diverse Patient Populations: Clinical trials often exclude a vast majority of the patient population, limiting the generalizability of findings.
- Vast Amount of Unresearched Combinations: The immense number of possible molecule-disease combinations leaves 99.9999998% of potential knowledge undiscovered.
- Bias Towards Rejecting Effective Treatments: Cognitive biases and personal risk aversion among regulators result in a tendency to reject potentially life-saving treatments.
To overcome these perverse incentives and biases, implementing a Decentralized Autonomous Organization (DAO) regulatory body is proposed. This would distribute responsibility among a large group of experts, mitigating individual risk and bias, and fostering a more balanced, efficient, and innovative drug approval process. 👉 Learn More
¶ Ingredients of the dFDA
¶ 1. Board of Directors
- Credibility: Cultivate a board that embodies trust, expertise, and a stellar reputation in the health and tech industries.
- Reach: Leverage the network of the board to access a wealth of data, resources, and strategic partnerships.
- Funding: Explore innovative funding avenues, including health-focused prizes like the XPRIZE, grants, and private investments.
- Proven Track Record: Highlight and leverage previous successful initiatives led by board members to instill confidence in stakeholders.
- Mission Alignment: Ensure every board member is passionately aligned with the mission, understanding their role in this transformative journey.
- Incentives: Clearly articulate the value proposition for board members, showcasing the impact and personal/professional benefits of their involvement.
¶ 2. dFDA Coordination Platform
The primary initial deliverable a coordination platform. This platform would act as the nexus for facilitating cooperation, communication, and collaborative actions among various stakeholders. It's designed to harness the collective capabilities of existing entities towards achieving the shared vision of accelerated clinical discovery and better health outcomes.
The coordination platform should ideally provide:
-
Communication Channels: Enable seamless communication among stakeholders, fostering a community of shared knowledge and goals.
-
Resource Sharing Mechanisms: Facilitate the sharing of data, technologies, expertise, and other resources among partners.
-
Decentralized Collaborative Workspaces: Provide tools and spaces for collaborative research, data analysis, and project development.
-
Partnership Agreements: Streamline the formation and management of partnerships, ensuring clarity on roles, responsibilities, and contributions.
-
Project Management Tools: Offer tools for planning, tracking, and managing collaborative projects, ensuring alignment and progress towards shared goals.
-
Knowledge Repository: Create a centralized or federated repository for collective knowledge, research findings, and best practices.
-
Legal and Regulatory Guidance: Provide guidance on navigating the legal and regulatory landscape for collaborative endeavors, ensuring compliance and mitigating risks.
-
Impact Tracking: Implement tools for monitoring, evaluation, and reporting on the impact and outcomes of collaborative projects.
The coordination platform encapsulates a digital environment where stakeholders can come together to synergistically work towards the broader objectives of the dFDA initiative. Through this platform, the barriers to collaboration are minimized, and the pace of innovation and discovery is expected to accelerate, aligning with the overarching mission of maximizing human lifespan and minimizing net suffering.
¶ 3. Digital Twin Safes / PersonalFDA Nodes
A tool for self-sovereign storage of personal data that enables effortless data sharing with clinical safety and efficacy studies.
Features
- Data Import: Create seamless mechanisms for importing existing health data while ensuring privacy and security. Import data from all your apps and wearables, so you can centrally own, control, and share all your digital exhaust.
- Data Encryption: Implement robust encryption protocols to safeguard sensitive health data.
- Sync to Trusted Instances: Establish secure channels for data synchronization, ensuring integrity and reliability.
- Federated Learning with Homomorphic Encryption: Innovate in secure data analysis, allowing for meaningful insights without compromising data privacy.
- Data Gems NFTs - Data sets can be encrypted and stored in a decentralized manner generating a Data Gem NFT that can be sold on data exchanges granting the possessor access to the data set.
- Digital Twin Skeleton Key NFT - This key gives you the ability to mint Data Gem Data Access NFTs using your imported data.
- The Human File System Protocol SDK - A Simple API for Patient-Controlled Health Data Aggregation, Sharing, and Monetization. Also standard protocol for personal data exchange between studies, apps and devices.
Potential Implementations, Components or Inspiration

¶ 4. dFDA Wiki
- Knowledge Base: Inspiration could be taken from the Psychonaut Wiki. It's a modified version of MediaWiki with additional quantitative metadata storage regarding the pharmacokintics of various substances. This could be expanded to document the quantitative effects of every factor on specific health outcomes.
- Editing Authorization: Implement a robust authorization mechanism to maintain content integrity and trustworthiness.
- AI-Powered Data Population: Leverage AI to efficiently populate the wiki with initial research and data.
- Data Silos Directory: Compile a comprehensive directory of existing data sources, facilitating integration with the Digital Twin Safe.
- Reputation Scoring: Develop a transparent and reliable reputation-weighted voting system for intervention approval.
- Comparative Policy Analysis - Aggregate existing approval and certification data from existing national regulatory bodies
- Food and Drug Outcome Labels - Ultimately, the most useful output of a decentralized FDA would be Outcomes Label that list the degree to which the product is likely to improve or worsen specific health outcomes or symptoms. These are derived from real-world data (RWD) and subject to Futarchical-weighted review by the board members of the dFDA.
- Publish Meta-Analyses - Generate meta-analyses from all completed studies at ClinicalTrials.gov
- Certification of Intervention Manufacturers/Sources via a Decentralized Web of Trust derived from end-user data and reviews traced back though an NFT-tracked supply chain
- Intervention Ranking - Elevate the most promising yet little/known or researched treatments
- Decentralized Clinical Trials - Not only would this increase knowledge but also access and availability of new and innovated treatments to those who need them urgently.
Potential Implementations, Components or Inspiration
¶ Roadmap
¶ Milestone 1: Establish Foundation
Objective: Lay down the groundwork for the Decentralized FDA, defining its scope, audience, and core values.
Tasks:
- Define Project Scope and Goals: Clearly outline what the Decentralized FDA aims to achieve, its target audience, and its core mission.
- Framing and Naming: Develop a strong framing narrative and decide on a compelling name for the initiative.
- Identify Target Audience: List out the potential board members, disease advocacy organizations, and other key stakeholders.
- Initial Stakeholder Engagement: Begin outreach to potential board members and key stakeholders to introduce them to the project and gauge interest.
¶ Milestone 2: Building the Board of Directors
Objective: Assemble a diverse and influential Board of Directors to guide and support the initiative.
Tasks:
- Credibility and Reach: Identify and onboard individuals with credibility, reach, and a passion for the project’s mission.
- Funding Strategies: Develop strategies for funding, exploring options like health-focused prizes, grants, and private investments.
- Define Value Proposition for Board Members: Clearly articulate what’s in it for them, outlining the impact and benefits of their involvement.
¶ Milestone 3: Collaborations and Partnerships
Objective: Identify and engage with entities already working in similar domains to foster collaboration and knowledge sharing.
Tasks:
- Research Potential Collaborators: Identify entities, initiatives, and experts working on similar projects.
- Initiate Outreach: Reach out to potential collaborators to explore partnership opportunities.
- Develop Collaborative Projects: Work on joint initiatives, sharing knowledge and resources for mutual benefit.
¶ Milestone 4: Developing the Digital Twin Safe
Objective: Implement the Digital Twin Safe with Locally Runnable Nodes, ensuring a secure and efficient data environment.
Tasks:
- Data Import Mechanisms: Develop tools and protocols for importing existing health data.
- Implement Data Encryption: Ensure robust encryption for data at rest and in transit.
- Sync to Trusted Instances: Establish secure and reliable data synchronization channels.
- Federated Learning with Homomorphic Encryption: Innovate in secure data analysis, allowing for insights without compromising privacy.
¶ Milestone 5: Creating the dFDA Wiki
Objective: Develop and launch the initial version of the Decentralized FDA, populated with initial data and research.
Tasks:
- Develop the Knowledge Base: Utilize a modified version of the Psychonaut Wiki as a starting point.
- Editing Authorization Mechanism: Implement a secure and reliable editing authorization process.
- AI-Powered Data Population: Leverage AI to populate the wiki with initial data and research.
- Compile Data Silos Directory: Create a comprehensive directory of existing data sources for integration.
¶ Milestone 6: Continuous Improvement and Scaling
Objective: Continuously improve the Decentralized FDA, expanding its reach, content, and impact.
Tasks:
- Gather User Feedback: Actively seek feedback from users and stakeholders to identify areas for improvement.
- Implement Updates and Enhancements: Regularly update the platform, adding new features and content based on user feedback and evolving needs.
- Scale Operations: Expand the reach of the Decentralized FDA, engaging with more stakeholders and increasing its impact over time.
¶ Benefits
¶ For Individuals
- eBay for health data - You can earn magic internet money by selling your data regarding symptoms, treatments, and
factors to pharmaceutical companies, insurance companies, and other data buyers - Control access and use of your data through fine-grained permissions
- Continuously monitor and audit the data you provide to other organizations
¶ For Health Apps
- Connected real-world data yields better insights for your users
- Apps that embed the exchange in their app earn a 0.5% transaction fee for each data sale
- Connect to third-party sources to enrich your data, or easily connect to a user's existing data
¶ For Pharmaceutical Companies
- Conduct long-term safety and effectiveness studies by linking their clinical trial data to medical claims and
electronic health record data - Refine models for finding rare disease patients by linking diagnostic lab, genomic, and imaging data
- Discover new therapeutic candidates with connected data
¶ For Insurers
- Improve value-based care analytics and sharpen total cost of care estimates by linking to EHR and clinical data
- Connect to the nation's largest ecosystem of health data
- Hone risk adjustment factor calculations by linking claims to social determinant's data, to properly estimate the true
cost of patient care
¶ Conclusion
The dFDA could provide innovative tools, resources, and frameworks, the dFDA empowers Contract Research Organizations, Certifying Agencies, and Regulatory Agencies to perform their roles more effectively, accelerating safety and innovation.
¶ More Info
- dFDA Governance Protocol - By implementing a continuous improvement process and a Futarchical Voting mechanism, the dFDA aims to enhance the quality of drug approvals and health policies.
- Regulatory Enhancements - The ideal framework for drug approvals aims to harness the collective intelligence of a diverse and knowledgeable crowd, ensuring rigorous, transparent, and inclusive decision-making processes and algorithmic optimization of outcomes using quantititative cost benefit analysis of every intervention for each condition
- Support of Real World Evidence - Large-scale efficacy trials based on real-world evidence, utilized prior to the pharmaceutical industry-driven randomized controlled trials mandated post-1962, were more effective in improving health outcomes, as evidenced by a consistent increase in human life expectancy during their implementation.
- dFDA Legal Structure - In considering the optimal legal structure for the dFDA, it is crucial to evaluate the advantages, disadvantages, and suitability of various options to ensure that the organization can fulfill its mission effectively while remaining compliant with legal and regulatory requirements.
- Life Force NFTs - Gamification to Incentivize Healthy Behaviour with Life Force Score. NFT's with an avatar and Life Force Score metadata updated daily based on performing healthy activities, such as having a good sleep schedule, hydrating, and exercising.
- DeSci Exchange - A free market for personal data in the form of an embeddable SDK with a configurable transaction fee for the apps embedding it.