¶ Incentive Alignment
Fully realizing the potential of the personalized preventative medicine of the future will require incentivizing cooperation between the following stakeholders:
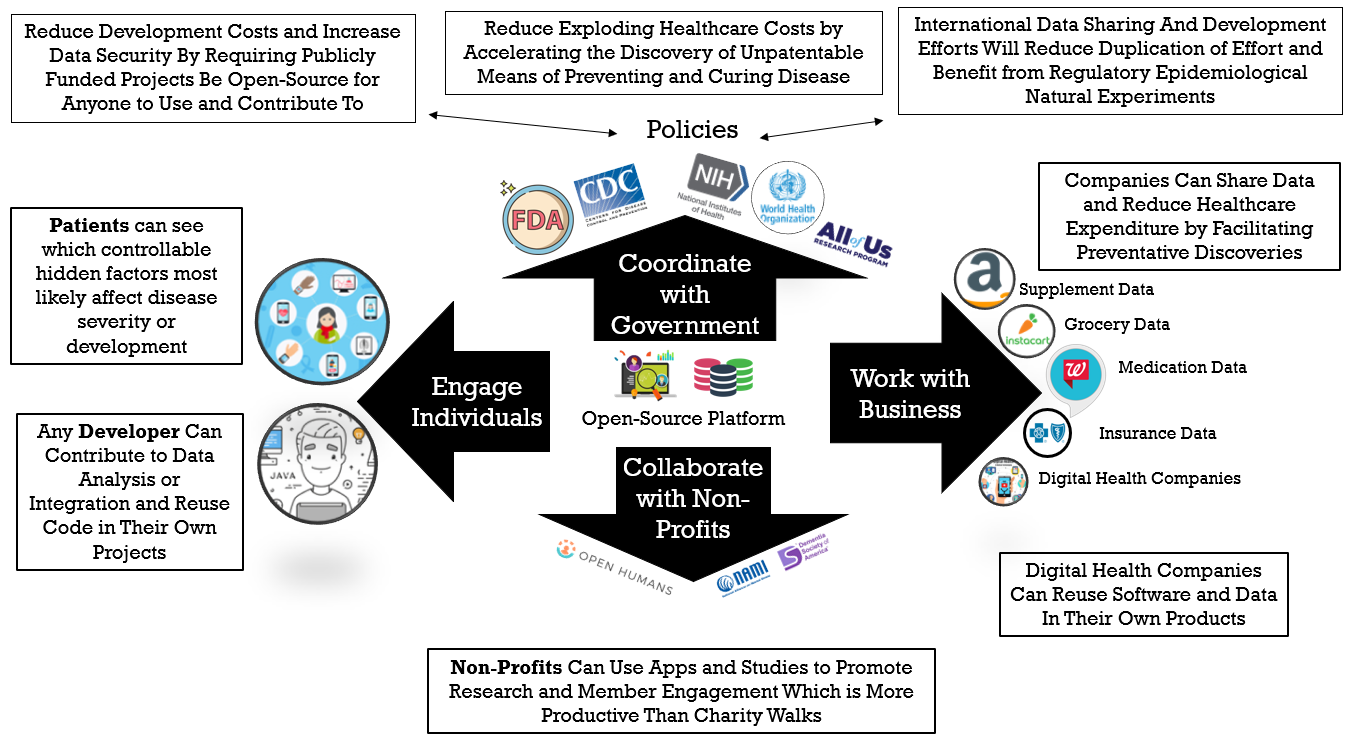
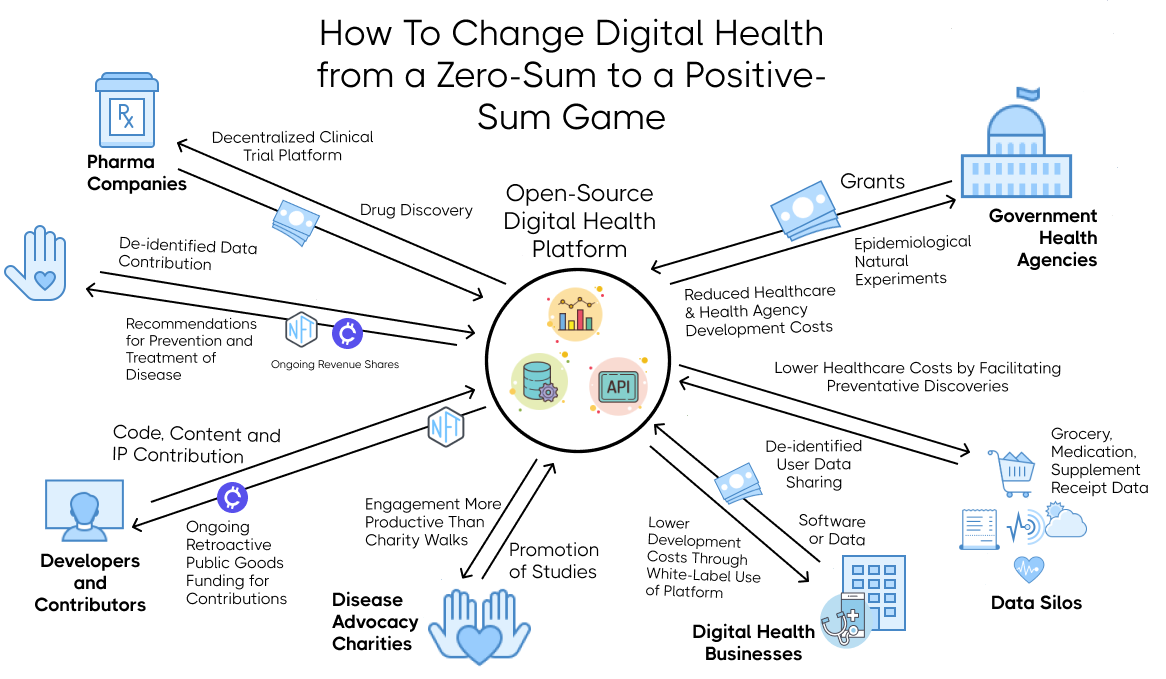
This illustrates the flow of value between different stakeholders. As opposed to traditional zero-sum games, we provide a way for each self-interested party to derive more value from participation than they have to put into it.
¶ 1. Patients
Incentives for Patients to share their de-identified data will include:
- Actionable ways to prevent and mitigate chronic illnesses.
- The ability to license and earn a share of income for use of their data for research and development by pharmaceutical companies and other businesses. This will be achieved by encoding the user data using non-fungible tokens (NFTs) and issuing them to the user. The NFTs will be linked to the user's cryptographic wallet address. Using a smart contract, the user will receive an ongoing royalty share of the profits for any product that was developed using their data for research and development.
¶ 2. Businesses Housing Data Silos
Businesses housing data silos include health insurers, pharmacies, grocery delivery services, digital health apps, hospitals, etc. These will be incentivized to allow individuals to easily share their data via a well-documented OAuth2 API by:
- A share of income for use of their data for research and development.
- An on-site instance of the OAuth2 server to retrieve required data from their on-premise databases.
- An eventual reduction in their employee healthcare costs (one of their largest expenses) by resulting from the discovery of new ways to prevent and mitigate chronic illnesses.
¶ 3. Digital Health Businesses
On top of the incentives for businesses listed above, the following incentives will be provided to digital health businesses which enable data sharing:
- A license to use a white-labeled version of the framework. This will dramatically reduce the costs of software development. These reduced costs will allow them to focus on innovating their unique value proposition, making them more competitive in the market.
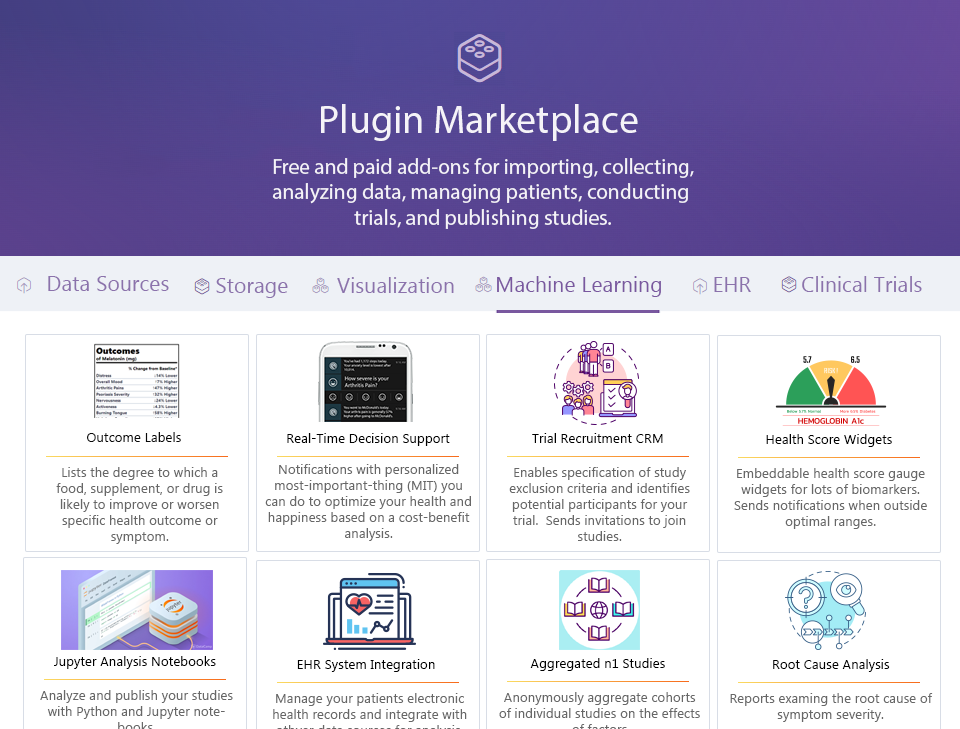
- Massive free marketing exposure through company branded plugins in the Plugin Marketplace.
- Revenue derived through subscription or licensing agreements for usage of their plugins in the Plugin Marketplace.
¶ 4. Disease Advocacy Non-Profit Organizations
Disease advocacy non-profits will be incentivized to promote observational studies through the anonymous donation data by their members by:
- Accelerated furtherance of their mission to reduce the incidence of chronic illnesses.
- A new method of member engagement more motivating and productive than the traditional charity walk.
¶ 5. Government Health Agencies
Governments will be incentivized by:
- A reduction in government healthcare costs due to the discovery of new ways to prevent and mitigate chronic illnesses.
- Furtherance of their stated reason for existence to protect and promote the general welfare. General welfare is defined as the overall health and happiness of the population.
- Their duty to protect the rights of individuals' data. To fulfill this, they must require businesses in possession of it to give them the ability to access and share their data via a well-documented OAuth2 API
- Cost-savings from using open-source software. All publicly funded digital-health software projects should be free, secure, and open-source. Currently, the majority of government contracts go to closed-source and proprietary software. This leads to massive waste as governments around the world are paying to reinvent the wheel instead of sharing the costs. Shockingly, there is even a great deal of wasted money on duplicated software contracts between different agencies within the same governments.
- Require international cooperation for all public health efforts to reduce wasted duplication of effort and take advantage of natural experiments resulting from differing public health regulations between nations.
- Epidemiological discoveries from allowing citizens to anonymously share their data in a global database. This will enable us to take advantage of natural experiments resulting from differing public health regulations between nations. For instance, 27 countries have banned the use of the pesticide glyphosate due to concerns about the health effects. If no overall change in the health of the populations is observed, it will suggest that the health concerns may be unfounded.
¶ 6. Contributors to the Development of the Platform
Citizens are incentivized to contribute to the development of the platform by:
- Gitcoin Bounties for specific tasks
- Encoding git commits with NFTs entitling the developer to ongoing retroactive public goods funding royalties in proportion to their contributions.
¶ Empowering External Stakeholders
The Decentralized FDA (dFDA) aims to act as a pivotal tool and resource, empowering a range of external organizations engaged in biomedical research, safety assessment, and regulatory processes. Rather than directly filling the roles of a Contract Research Organization (CRO), Certifying Agency, or Regulatory Agency, the dFDA seeks to support and enhance the capabilities of entities that operate in these domains.
¶ 1. Support for Contract Research Organizations (CROs)
Facilitating Research and Trials:
- Research Tools and Resources: Provide innovative tools and decentralized frameworks that CROs can utilize to conduct research, focusing on early-stage development and replication experiments.
- Decentralized Trial Infrastructure: Offer a robust infrastructure to facilitate the execution of clinical trials in a decentralized manner, whether in specific locales like Prospera or globally distributed.
- Collaboration and Integration: Encourage knowledge sharing and integration between CROs, DAOs, and corporations, fostering a collaborative ecosystem for biomedical innovation.
¶ 2. Enhancing the Capabilities of Certifying Agencies
Comprehensive Evaluation and Trust Building:
- Gradient Rating Tools: Supply advanced tools and methodologies to enable certifying agencies to implement gradient safety and efficacy ratings, moving beyond binary pass/fail assessments.
- Experiment Approval and Oversight: Provide resources and frameworks to assist Institutional Review Boards (IRBs) in approving and overseeing proposed experiments, ensuring ethical standards and participant safety.
- Certification and Trust Mechanisms: Facilitate the certification of named and pseudonymous participants in DeSci/Biomedicine, contributing to the development of a secure and reliable web-of-trust.
¶ 3. Augmenting Regulatory Agency Functions
Streamlining Processes and Ensuring Accountability:
- Web3 API for Marketplaces: Develop a comprehensive Web3 API to streamline the integration of regulatory data and certifications with various marketplaces.
- Certification Support for the Treatment Supply Chain: Provide tools and frameworks to assist in the certification processes of producers, distributors, and recipients of treatments.
- Knowledge Graph and Data Utilization: Supply the necessary resources to build and maintain a knowledge graph from 'confirmed' treatment recipient data, enhancing transparency and informed decision-making.
- Mechanisms to Address Non-Compliance: Offer solutions to identify and take appropriate actions against 'bad actors' within the ecosystem, ensuring the integrity of the biomedical landscape.